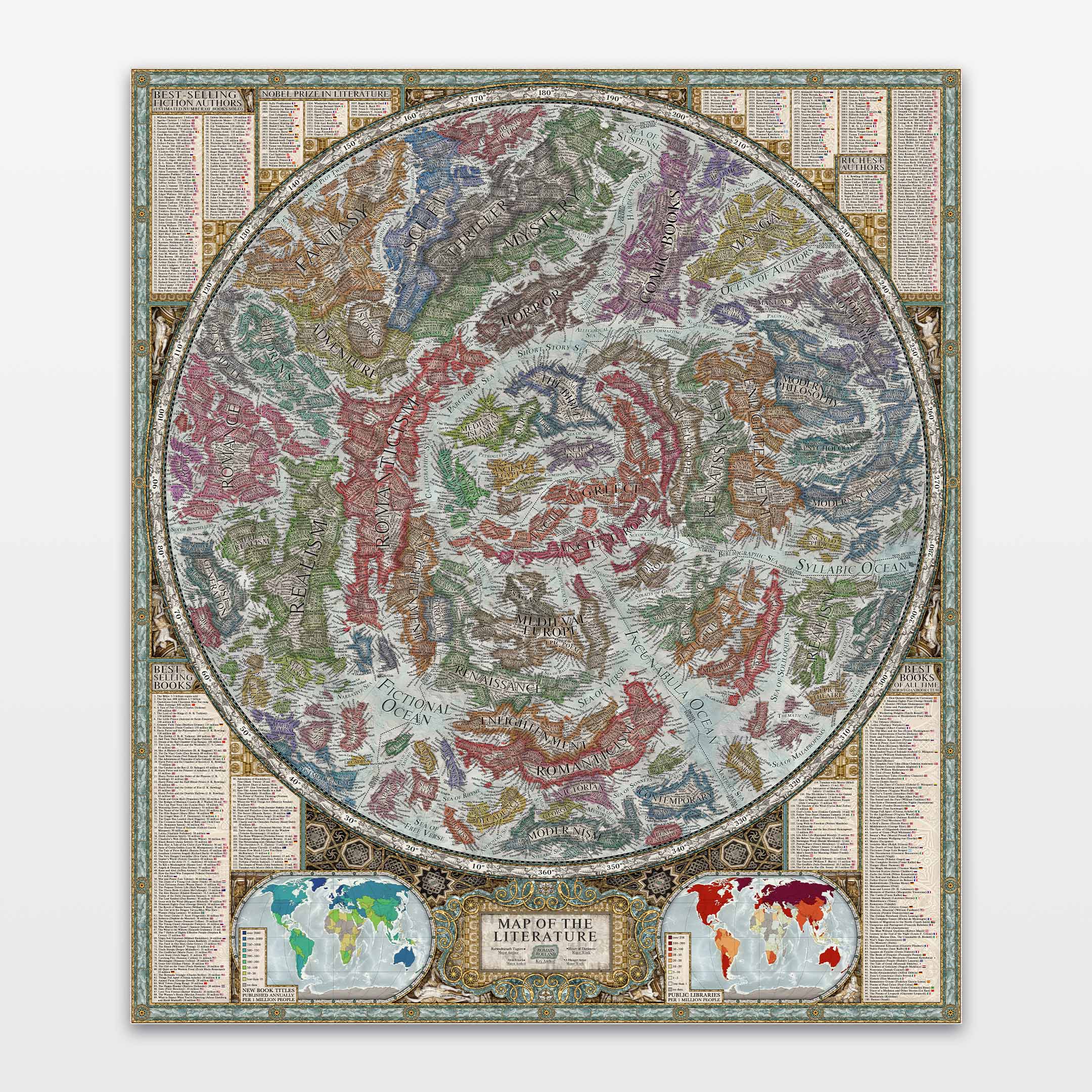
This 2025 poster infographic showcases an up-to-date periodic table of the elements, visualizing all 118 currently known chemical elements, from hydrogen to oganesson. Included are many of their characteristics and properties, such as atomic weight, melting and boiling points, density, electron structure, crystal structure, Mohs hardness, spectral emission lines, oxidation states, and more.
On this periodic table, you will find photographs and illustrations pertaining to each element, if possible showing it in its pure state; in the case of many radioactive elements, minerals containing trace quantities of them.
Surrounding it, you will find a lot of useful information, including the origins of various chemical elements, shapes of electron orbitals, as well as the abundance, discovery, naming origins, annual production and monetary value of elements, a diagram of the states of matter, how the different elements making up our planet formed, and structures of several hundred important and interesting organic and inorganic molecules.
The periodic table of the elements arranges all known chemical elements into rows (periods) and columns (groups), and is widely used in chemistry, physics, and other sciences. Its arrangement showcases the “periodic law,” which tells of an approximate recurrence of many properties, such as density, melting point, reactivity, or electrical conductivity, among elements sharing the same column. This recurrence exists because of their similar outer electron configuration.
Several trends are evident on the periodic table. One of them is electronegativity — the atom’s ability to attract electrons when forming a chemical bond, which also corresponds to bond energy and the element’s reactivity. It increases from the bottom left to the top right, with francium having the lowest electronegativity and fluorine having the highest. On the contrary, atomic radius and density decrease from the bottom left to the top right — atoms of the noble gases are over twice as small in radius as those of the alkali metals that follow them.
As we go from right to left, elements increasingly gain metallic character, from non-metals to weakly metallic metalloids, followed by metals. Melting and boiling points of elements, with some exceptions such as carbon, peak in the center of the periodic table with tungsten, tantalum, and rhenium, and decrease in either direction. The first periodic table to become widely accepted was that created in 1869 by the Russian chemist Dmitri Mendeleev, who reportedly envisioned it in a dream, arranging chemical elements by their atomic mass. At the time, dozens of chemical elements were not yet known, so he left gaps in his periodic table, successfully predicting some of the properties of undiscovered elements such as scandium, gallium, and germanium. The periodic table only reached its modern recognizable shape in 1945, when Glenn T. Seaborg placed the actinides in their proper position below the lanthanides.
Every period in the periodic table corresponds to a new outer electron shell, with the maximum being seven distinct shells. Chemical elements with the same number of electrons in their outer electron subshell can be found in the same column (group).
Each chemical element has its unique atomic number, representing the number of protons found in its nucleus. However, most elements also have several different isotopes, differing in the number of neutrons. Some elements have only a single stable isotope, many have multiple, but most isotopes are radioactive, cannot be found in nature, and decay quickly. Of all the currently known elements, 94 occur naturally, 83 being stable, while the remaining 24, from americium to oganesson, can only be synthesized using particle accelerators. No element heavier than einsteinium (99) has ever been amassed in macroscopic quantities, and half-lives of all elements beyond copernicium (112) can only be measured on the scale of seconds. Of the 94 naturally occurring elements, nine are gaseous at room temperature, two occur in a liquid state, and the remaining 83 are solid. Atoms of chemically pure elements can bond to each other in multiple different arrangements, resulting in a number of different “allotropes.” For instance, carbon can bond in a lattice of hexagonal sheets as graphite, in a cubic lattice as diamond, or chaotically to form amorphous carbon in coal. Allotropes can differ wildly in their hardness, density, and other properties.
With gratitude to: